

VSEPR focuses on the positions taken by the groups ofĮlectrons on the central atom of a simple molecule. These ideas have been explored and have resulted in a theoryįor molecular geometry known as Valence Shell Electron-Pair The position of the atoms is dictated by the position of theīonding groups of electrons. To arrive at geometries which result from valenceĮlectrons taking up positions as far as possible from each other. By recognizing that electrons repel each other it is possible It will exhibit this particular geometry. As indicated in Table I, any compound containing aĬentral atom with four bonding groups (pairs) of electrons around The natural arrangement is as a tetrahedron. When four balloons of the same size are tied together This was seen in the 'balloon' example we used The four bonds positioning themselves so as to minimize the The shape of this molecule is a result of the electrons in This geometry is a direct result of the repulsionĮxperienced by the four groups of bonding electrons. See is the only possible shape for a central carbon atom withįour bonds. Molecule has to have this 3-dimensional arrangement. This ball and stick model does not adequately represent why the The actual molecular geometry is not flat, but is tetrahedral. What does this do to our geometry? Lets rotate this molecule to see what has happened. However, the actual bond angles in this molecule are 109.5ĭegrees. The Cl-C-Cl bond angles appear to be 90 degrees. This is a nice representation of a two dimensional, flat
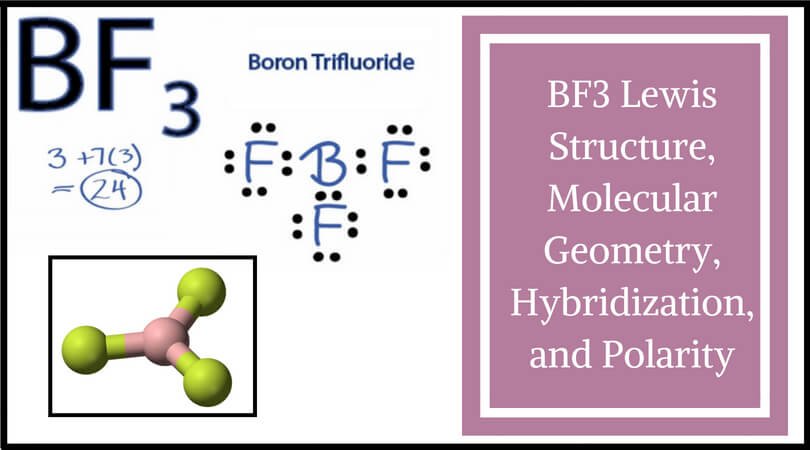
But this drawing does not tell us about the shape of Is the carbon is the central atom and the four chlorine atoms are The arrangement of the atoms is correct in my structure. Each chlorineĪtom has three nonbonding pairs of electrons. In CCl 4 the centralĬarbon atom has four bonding groups of electrons. Bonding electrons, which are shared by a pair of atomsĪnd nonbonding electrons, which belong to a particular atom butĭo not participate in bonding. Notice that there are two kinds of electron groups in this We canĭraw the Lewis structure on a sheet of paper. Lets consider the Lewis structure for CCl 4. Geometry is tetrahedral the bond angle is around 105 degrees. For bent molecular geometry when the electron-pair The bond angle is slightly less than 109.5 degrees, around 107ĭegrees. Geometry is trigonal planar the bond angle is slightly less thanġ20 degrees, around 118 degrees. Note: for bent molecular geometry when the electron-pair The table below summarizes the molecular and electron-pair geometriesįor different combinations of bonding groups and nonbonding pairs of electrons The Lewis structure and determine the number of bonding groups of electronsĪnd the number of non-bonding pairs of electrons on the central atom, then use That to determine the shape (molecular geometry) of a molecule you must write Notice that there are several examples with the sameĮlectron-pair geometry, but different molecular geometries. If askedįor the electron-pair geometry on the central atom we must respond with theĮlectron-pair geometry. The shape of a molecule we must respond with a molecular geometry. The molecular geometry is the shape of the molecule. To the bond angles of between a terminal-central-terminal atom in a compound. The electron-pair geometry provides a guide Molecular geometry is the name of the geometry used


Name of the geometry of the electron-pair/groups/domains on the central atom, whether theyĪre bonding or non-bonding. In this case thereĪre three groups of electrons around the central atom and the molecualr geometry When a central atom has two terminal atoms bonded by single bonds and a terminalĪtom bonded with two pairs of electrons (a double bond). The term bonding groups/domains (second from the left column) is used in the columnįor the bonding pair of electrons. On an individual atom that are not shared with another atom. Non-bonding pairs of electrons are those pairs of electrons Of electrons are those electrons shared by the central atom and any atom to Of bonding pair of electrons and non-bonding pairs of electrons. The table below contains several columns. To use the model we will have to memorize a collection Model called the Valence Shell Electron-Pair Repulsion (VSEPR) model that isīased on the repulsive behavior of electron-pairs. Of simple molecular (covalent) compounds and polyatomic ions. Molecular Geometry Molecular Geometry VSEPR At this point we are ready to explore the three dimensional structure


 0 kommentar(er)
0 kommentar(er)
